Clock. bio is on a mission to decode human rejuvenation!
Stem cells. Seldom, if ever, has a medical procedure seemed so promising – and so controversial. Stem cell therapy research trials are underway to attack head-on 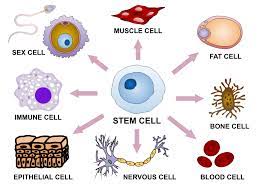 such killer neurodegenerative diseases like Alzheimer’s Disease, Parkinson’s disease, cardiovascular disease, Multiple Sclerosis, rheumatoid arthritis, spinal cord injuries, and amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease.
such killer neurodegenerative diseases like Alzheimer’s Disease, Parkinson’s disease, cardiovascular disease, Multiple Sclerosis, rheumatoid arthritis, spinal cord injuries, and amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease.
These afflictions are extremely challenging to combat; countless lives are lost and will be lost until a new approach like stem cell therapy is perfected.
One study concluded that people experiencing heart failure showed some improvement two years after a single-dose administration of stem cell therapy. However, the effect of stem cell therapy on the heart is unclear, and research is continuing.
Another study concluded that stem cell therapies could lead to personalized diabetes treatment. That Study’s author, Jeffrey R. Millman — an assistant professor of medicine and biomedical engineering at the Washington University School of Medicine in St. Louis, MO — said, “What we’re envisioning is an outpatient procedure in which some sort of device filled with the cells would be placed just beneath the skin.” Millman hopes that these stem cell-derived beta cells could be ready for human trials within 3–5 years.
The Food and Drug Administration (FDA) has only approved the treatment for specific cancers and conditions affecting the blood and immune system. Stem cell therapy is FDA-approved to treat the following conditions: Leukemia, Lymphoma, Neuroblastoma, and Multiple Myeloma. The therapy can also be used to lower the risk of infection after stem cell transplantation in people with blood cancers.
Unlimited Possibilities
The excitement about stem cell therapy is because stem cells are the body’s repair team. This is due to the difference between stem cells and ordinary cells.  Stem cells divide and renew themselves forever, transforming into the kind of cell that needs rejuvenation.
Stem cells divide and renew themselves forever, transforming into the kind of cell that needs rejuvenation.
To word it another way, stem cells are the body’s repairmen.
So why the controversy?
There are a few reasons and one significant understanding. First, scientists are concerned that tampering with these cells as part of stem cell therapy could cause cancerous tumors.
Second, a system must be in place to ensure donors of their cells understand the procedure and give informed consent to have their iPS cells extracted.
The third reason for the controversy is ethical. In the 1990s, scientists were using embryonic cells for medical research. This created a firestorm since extracting them meant destroying the embryo and led to an intense, emotional, and religious debate about what means the beginning of human life.
For some, life starts when a baby is born; others disagree, insisting that life begins when an embryo develops into a fetus. Meanwhile, other people believe that human life begins at conception, which makes an embryo a living, breathing person entitled to all humans' protection.
Former U.S. president George W. Bush fell strongly into the latter camp and stopped government funding for human stem cell research in 2001. Former U.S. president Barack Obama revoked the ban, but recent scientific developments have made this issue far less contentious.
Here’s why. By the mid-2000s, researchers switched from using embryonic stem  cells to human induced pluripotent stem cells (hiPSCs). This change is significant since pluripotent stem cells are reprogrammed to turn adult cells into stem cells, removing the ethical concerns of embryonic stem cells. However, other concerns related to using iPS cells still exist.
cells to human induced pluripotent stem cells (hiPSCs). This change is significant since pluripotent stem cells are reprogrammed to turn adult cells into stem cells, removing the ethical concerns of embryonic stem cells. However, other concerns related to using iPS cells still exist.
There is also a misunderstanding about embryonic stem cells. They can be harvested from the baby's umbilical cords and placentas that are tossed in the trash after birth. This is even though these cells are far more potent than the aging stem cells they can replace.
So, no more extractions of embryonic stem cells from babies.
As a result of these developments, the age of stem cell therapy is rapidly dawning on the medical horizon. And the biotech company Clock. bio is prepared to lead the charge.
Clock. bio, a longevity biotech company developing regenerative medicines leveraging the ability of human pluripotent stem cells to prevent and treat age-related diseases, today announced it has launched out of stealth after reaching proof-of-concept with $4 million in funding.
The company’s immediate scientific goal is to decode all rejuvenation programs present in human cells and then build a map of disease and rejuvenation targets for clinical interpretation.
“We have a bold mission of extending human health span by 20 years based on biomarkers of aging in a Phase 3 trial by the end of this decade. Embryonic stem cells hold the key to unlocking rejuvenation biology,” said Mark Kotter, clock.bio’s founder.
“We believe that a comprehensive understanding of all relevant repair processes is the best starting point for designing regenerative and healthspan-increasing treatments.”
Clock. bio plans to extend and ramp up the quality of life by reversing the detrimental effects aging inflicts on our cells by using the regenerative capabilities of human pluripotent stem cells. The company has developed an aging model that speeds up aging in human induced pluripotent stem cells (hiPSCs) and triggers their self-rejuvenation mechanism.
CRISPR screens on significant samples of these cells allow for identifying genes relevant to cell rejuvenation. The company’s strategy includes proprietary aging interventions for iPSCs, unbiased screens for rejuvenation biology, identification and confirmation of rejuvenation targets, and therapeutic lead prioritization and translation into clinical applications.
Markus Gstöttner will be taking over as clock.bio’s CEO. Previously, he co-founded Meatable, a cultured meat company that uses iPSC and opti-ox technologies, which he started with Mark Kotter and others. Building on this partnership, Markus will join the company to drive Clock.bio’s mission of finding  new treatments for age-related diseases; he is now the EIR at BlueYard Capital, with a prior background in public service for the Austrian government, management consulting at McKinsey, and development economics (LSE/J-PAL).
new treatments for age-related diseases; he is now the EIR at BlueYard Capital, with a prior background in public service for the Austrian government, management consulting at McKinsey, and development economics (LSE/J-PAL).
“Somatic cells age but cannot rejuvenate. Pluripotent stem cells are the only cells in the human body that can rejuvenate. Our unique approach is to force-age human iPSCs and trigger their self-rejuvenation mechanism,” said incoming CEO Gstoettner.
“Human aging is a complex process that is difficult to model in cell culture. Clock.bio’s model delivers ‘aging in a dish,’ where unbiased CRISPR screens reveal the genes that cause aging/rejuvenation and thus uncover drivers.”
Clock. bio says it is on track to decode the biology of human rejuvenation across the entire genome in 12 months. The result should be a comprehensive atlas of disease and rejuvenation targets for clinical translation and clock.bio’s vision is to use this technology to enable a comprehensive decoding of rejuvenation biology to create novel treatment approaches.
The company aims to boost healthspan and lifespan vision by developing new treatments to prevent and treat age-related diseases by decoding the rejuvenation programs in human cells.
Their research has found that hiPSCs can stay young and rejuvenate when forced to age. They use the ‘resetting the clock’ power in hiPSCs to find new treatments for age-related diseases.
Bio. clock has developed an aging model that force-ages hiPSCs to activate their self-rejuvenation process and uses this knowledge to create fresh treatment approaches.
This is just one of several exciting breakthroughs in the field of life extension through biotechnology. Stay tuned for more!
References
https://longevity.technology/news/clock-bio-launches-to-decode-rejuvenation-biology-across-human-genome/
https://clock.bio/

- Human Growth Hormone Injections In Cheyenne [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Madison [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Vancouver [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Tacoma [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Spokane [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Bellevue [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Richmond [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Portsmouth [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Norfolk [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Hampton [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Chesapeake [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Alexandria [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Montpelier [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Provo [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Waco [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Richardson [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Plano [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Pasadena [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Midland [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Mesquite [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In McKinney [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In McAllen [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Lubbock [Last Updated On: February 7th, 2024] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Lewisville [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Laredo [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Killeen [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Irving [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Garland [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Denton [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Carrollton [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Brownsville [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Beaumont [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Austin [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Arlington [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Amarillo [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Abilene [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Nashville [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Murfreesboro [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Memphis [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Knoxville [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Clarksville [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Chattanooga [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Erie [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Allentown [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Salem [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Portland [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Gresham [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Eugene [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Tulsa [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Norman [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Toledo [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Dayton [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Columbus [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Akron [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Paterson [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Reno [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Henderson [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Omaha [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Lincoln [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Billings [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Springfield [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Independence [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Columbia [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Jackson [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Rochester [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Warren [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Lansing [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Flint [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Worcester [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Springfield [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Lowell [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Cambridge [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Augusta [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Shreveport [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Lafayette [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Louisville [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Lexington [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Wichita [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Topeka [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Olathe [Last Updated On: February 7th, 2024] [Originally Added On: October 20th, 2018]

List of USA state clinics - click a flag below for blood testing clinics.
Word Count: 1225