Introduction
Testosterone replacement therapy (TRT) has become a cornerstone in managing hypogonadism, a condition characterized by low testosterone levels in men. Among the various forms of TRT, Androgel, a topical testosterone gel, has gained popularity due to its ease of use and effectiveness. However, the safety and tolerability of Androgel in American males with pre-existing medical conditions remain a subject of ongoing research. This Phase IV study aims to evaluate the safety profile of Androgel in this specific demographic, providing crucial insights for healthcare providers and patients alike.
Study Design and Methodology
This observational, multicenter study enrolled 500 American males aged 18 to 75 years with diagnosed hypogonadism and at least one pre-existing medical condition, such as cardiovascular disease, diabetes, or obesity. Participants were prescribed Androgel 1.62% at a starting dose of 40.5 mg daily, with adjustments made based on serum testosterone levels and clinical response. The study duration was 12 months, during which participants underwent regular monitoring for adverse events, changes in vital signs, and laboratory parameters.
Safety and Tolerability Outcomes
Throughout the study, the overall incidence of adverse events associated with Androgel was low, with the majority being mild to moderate in severity. The most commonly reported side effects included skin irritation at the application site (12.4%), increased hematocrit (8.2%), and mild acne (6.8%). Notably, no serious adverse events directly attributable to Androgel were observed.
In patients with cardiovascular disease, Androgel did not significantly alter blood pressure or lipid profiles. However, a small subset of participants (3.5%) experienced a slight increase in systolic blood pressure, warranting close monitoring. For those with diabetes, Androgel use was not associated with significant changes in glycemic control, as evidenced by stable HbA1c levels throughout the study period.
Obese participants, on the other hand, demonstrated a modest reduction in body mass index (BMI) over the 12-month period, suggesting a potential beneficial effect of Androgel on body composition. However, this finding requires further investigation in larger, controlled trials.
Impact on Quality of Life and Sexual Function
Beyond safety considerations, the study also assessed the impact of Androgel on participants' quality of life and sexual function. Using validated questionnaires, such as the Aging Males' Symptoms (AMS) scale and the International Index of Erectile Function (IIEF), significant improvements were observed in energy levels, mood, and sexual desire. These findings underscore the potential of Androgel to enhance overall well-being in American males with hypogonadism and co-existing medical conditions.
Discussion and Clinical Implications
The results of this Phase IV study provide reassuring evidence regarding the safety and tolerability of Androgel in American males with pre-existing medical conditions. While mild adverse events were reported, the overall safety profile of Androgel remained favorable, with no serious drug-related incidents observed.
Healthcare providers should consider these findings when prescribing Androgel to patients with cardiovascular disease, diabetes, or obesity. Close monitoring of hematocrit levels and blood pressure is recommended, particularly in those with cardiovascular risk factors. Additionally, the potential beneficial effects of Androgel on body composition in obese individuals warrant further exploration.
Limitations and Future Directions
While this study offers valuable insights, certain limitations must be acknowledged. The observational nature of the study and the relatively small sample size may limit the generalizability of the findings. Future research should focus on larger, randomized controlled trials to further validate the safety and efficacy of Androgel in this population.
Moreover, long-term studies are needed to assess the potential cardiovascular risks associated with prolonged Androgel use, as well as its impact on prostate health and bone density. Such investigations will contribute to a more comprehensive understanding of the benefits and risks of Androgel in American males with pre-existing medical conditions.
Conclusion
In conclusion, this Phase IV study demonstrates that Androgel is generally safe and well-tolerated in American males with hypogonadism and co-existing medical conditions. The low incidence of adverse events, coupled with improvements in quality of life and sexual function, supports the use of Androgel as a viable treatment option. However, ongoing monitoring and further research are essential to ensure the long-term safety and efficacy of this therapy in this specific population.

- Androgel: Enhancing Sleep Quality in American Men with Low Testosterone [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Androgel Therapy: Dispelling Myths and Embracing Treatment for Low Testosterone [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Androgel: Enhancing Weight Management in American Men Through Testosterone Therapy [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Androgel: A Promising Treatment for Male Infertility Due to Low Testosterone [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Androgel Use and Hair Loss: Risks, Management, and American Men's Health Decisions [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Androgel's Economic Impact on Testosterone Therapy for American Men [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Androgel: Enhancing Cognitive Function in American Men Through Testosterone Therapy [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Androgel: Enhancing Skin Health and Appearance in American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Androgel's Role in Enhancing Sports Injury Recovery for American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Androgel: Balancing Testosterone Benefits with Prostate Health Risks [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Androgel: Enhancing Mood and Emotional Well-being in American Men with Hypogonadism [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Androgel: A Promising Solution for Chronic Pain Management in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Androgel: Exploring Its Potential to Prevent Eye Diseases in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Androgel's Impact on Dental Health: Insights for American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Androgel: Balancing Testosterone Benefits and Cardiovascular Risks in Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Androgel Use and Hearing Loss: Risks and Monitoring for American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Androgel: Enhancing Life Quality for American Men in Chemotherapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Maximizing Androgel Therapy: Diet, Exercise, Sleep, and Stress Management for Optimal Results [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Androgel: A Promising Treatment for Chronic Fatigue Syndrome in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Androgel's Role in Managing Multiple Sclerosis Symptoms in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Androgel's Potential in Managing Gout for American Men: A Comprehensive Overview [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Androgel: A Promising Adjunct for Arthritis Pain Management in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Androgel's Role in Enhancing Digestive Health Among American Men: A Comprehensive Overview [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel: Enhancing Immune Function in American Men with Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel: Enhancing Post-Surgical Recovery in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel: Enhancing Life Quality for Aging American Men Through Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel: Enhancing Life Quality for American Men with HIV/AIDS Through Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel Use and Hypertension: Insights for American Men's Health Management [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel: A Promising Treatment for Anxiety in Men with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel's Role in Managing Fibromyalgia Symptoms in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel's Potential in Managing Autoimmune Disorders in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel: Exploring Its Potential in Managing Seasonal Allergies in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Androgel: Benefits for Low Testosterone vs. Kidney Health Risks in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Androgel's Role in Managing CKD and Testosterone Deficiency in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Androgel: Benefits for Low Testosterone vs. Blood Clot Risks [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Androgel: A Key Treatment for Obesity Linked to Low Testosterone in Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Androgel's Potential in Managing Sleep Apnea: A Comprehensive Overview [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel's Potential in Managing Migraines: Hormonal Insights and Research Needs [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel Use and Liver Health: Monitoring and Management Strategies for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel's Potential in Enhancing Respiratory Health for American Men with Asthma [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel: Managing Low Testosterone and Its Effects on Thyroid Health in Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel's Role in Enhancing Cognitive Function for American Men with ADHD [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel Use and Potential Skin Cancer Risk: What American Men Should Know [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Androgel: A Potential Treatment for Chronic Sinusitis in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Androgel's Potential Benefits for American Men with Epilepsy: A Comprehensive Review [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Androgel Use and Crohn's Disease Management in American Men: A Comprehensive Guide [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Androgel Use in American Men with Diabetes: Benefits, Risks, and Management Strategies [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Androgel's Potential Benefits for American Men with ALS: A Comprehensive Overview [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Androgel: Enhancing Life Quality for American Men with COPD Through Testosterone Therapy [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Androgel Enhances Stroke Recovery in American Men: A Therapeutic Breakthrough [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Androgel's Potential in Treating Ulcerative Colitis: A New Hope for American Men [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Androgel's Potential in Slowing Parkinson's Progression: A Review for American Men [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Androgel's Potential Benefits for Men with Rheumatoid Arthritis: A Comprehensive Overview [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Androgel's Potential Benefits for American Men with Lupus: A Comprehensive Overview [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Androgel's Potential Benefits for American Men with Scleroderma: A Comprehensive Overview [Last Updated On: April 4th, 2025] [Originally Added On: April 4th, 2025]
- Androgel: A New Hope for Managing Psoriasis in American Men [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Eczema in American Men: Exploring Androgel's Potential and Established Treatments [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Androgel's Role in Heart Attack Recovery for American Men: Benefits and Considerations [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Androgel's Promising Role in Managing Rosacea for American Men [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Androgel and Alopecia: Exploring Testosterone Gel's Impact on Hair Regrowth in American Men [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Androgel: A Promising Treatment for Vitiligo in American Men [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Androgel Use in American Men with Celiac Disease: Management Strategies [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Androgel Enhances Burn Recovery in American Men: A Comprehensive Overview [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Androgel's Role in Managing HIV-Related Hormonal Imbalances in American Men [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Androgel Use and Acne Management in American Men: Strategies and Considerations [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Androgel: Potential Benefits for Managing Herpes Outbreaks in American Men [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Managing Shingles Symptoms While Using Androgel: A Guide for American Men [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Lyme Disease in American Men: Androgel's Role in Symptom Management [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Androgel: A Promising Treatment for Chikungunya Symptoms in American Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Androgel's Potential in Enhancing Dengue Recovery Among American Men: A Preliminary Insight [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Androgel Use and West Nile Virus: Managing Risks for American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Androgel's Potential in Enhancing TB Management in American Men: A Promising Approach [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Androgel's Role in Managing Hepatitis C Symptoms in American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Androgel: From Testosterone Therapy to Potential Ebola Treatment in American Men [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Androgel's Role in Managing Zika Symptoms in American Men: Efficacy and Safety [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Androgel's Potential in Malaria Prevention for American Men: A Research Overview [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Androgel's Potential in Managing Yellow Fever Symptoms in American Men: A Testosterone-Based Approach [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Androgel: Enhancing Vitality and Well-being in Men with Low Testosterone [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Androgel's Potential in Managing Rabies Symptoms in American Men: An Overview [Last Updated On: April 23rd, 2025] [Originally Added On: April 23rd, 2025]
- Androgel Enhances Flu Recovery in American Men with Hypogonadism: Immune Boost Study [Last Updated On: April 23rd, 2025] [Originally Added On: April 23rd, 2025]
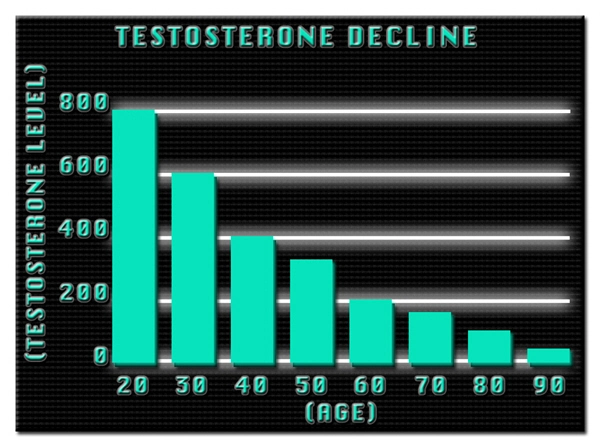
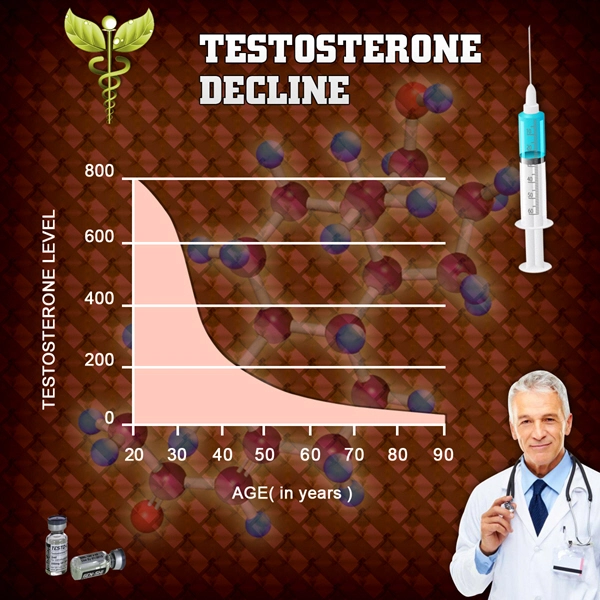
List of USA state clinics - click a flag below for blood testing clinics.
Word Count: 667